Evaluation of the effect of tooth and dental restoration material on electron dose distribution and production of photon contamination in electron beam radiotherapy
- 159 Downloads
Abstract
The aim of this study is to evaluate the effect of tooth and dental restoration materials on electron dose distribution and photon contamination production in electron beams of a medical linac. This evaluation was performed on 8, 12 and 14 MeV electron beams of a Siemens Primus linac. MCNPX Monte Carlo code was utilized and a 10 × 10 cm2 applicator was simulated in the cases of tooth and combinations of tooth and Ceramco C3 ceramic veneer, tooth and Eclipse alloy and tooth and amalgam restoration materials in a soft tissue phantom. The relative electron and photon contamination doses were calculated for these materials. The presence of tooth and dental restoration material changed the electron dose distribution and photon contamination in phantom, depending on the type of the restoration material and electron beam’s energy. The maximum relative electron dose was 1.07 in the presence of tooth including amalgam for 14 MeV electron beam. When 100.00 cGy was prescribed for the reference point, the maximum absolute electron dose was 105.10 cGy in the presence of amalgam for 12 MeV electron beam and the maximum absolute photon contamination dose was 376.67 μGy for tooth in 14 MeV electron beam. The change in electron dose distribution should be considered in treatment planning, when teeth are irradiated in electron beam radiotherapy. If treatment planning can be performed in such a way that the teeth are excluded from primary irradiation, the potential errors in dose delivery to the tumour and normal tissues can be avoided.
Keywords
Monte Carlo simulation Electron mode Tooth Dental restoration materialIntroduction
Dental restoration materials are used in dentistry for the restoration of tooth structure. There are four groups of materials that are currently used in dentistry for dental repair: metals, ceramics, polymers and composites. In radiotherapy of the head and neck, the teeth and mouth cavity are normally irradiated with a high radiation dose. As the compositions of teeth and such restoration materials are different from that of soft tissue, these materials are accounted as inhomogeneities, and when radiation interacts with these materials, secondary electrons are scattered in various directions, especially backward. These scattered electrons have an adverse effect on normal tissues, and there are evidences indicating that they may induce mucositis. Thus, teeth and dental restoration can change the dose distribution of the primary beam, resulting in high-dose irradiation of the adjacent mucosal tissues [1]. In various studies, the dose distortion in photon dose distribution due to the presence of high atomic number inhomogeneities have been evaluated [1, 2, 3, 4]. However, only a limited number of studies have been conducted on the effect of dental inhomogeneities on electron beam radiotherapy. In simulation studies, gold, titanium, amalgam, ceramic, composites and nickel–chromium alloy have been evaluated [1, 2, 3, 4, 5, 6].
In electron beam radiotherapy, when high-energy electrons interact with a high atomic number material, bremsstrahlung rays are produced. These bremsstrahlung radiations are accounted as photon contamination and are not incorporated in treatment planning calculations. Furthermore, as the range of these rays may be higher than those of the electrons, they may expose the farther tissues to unwanted radiations. In the study conducted by Reitemeier et al. [1], the absorption and backscattering effect of amalgam, gold alloy, pure titanium and an artificial material was evaluated following radiotherapy with 6 and 15 MV photons. For this purpose, MCNP-4C code was used, and the calculations were performed for a 20 × 20 cm2 field. The results revealed that backscattering from the dental restoration materials caused 170 % dose enhancement, while there was no dose enhancement at depths beyond the dental depth. Furthermore, dose enhancement proportionally increased with atomic number, and the attenuated radiations were less than the backscattered photons. Thus, shielding was suggested for patients with amalgam and gold restorations. Bjelkengren [2] used EGSnrc code for the calculation of electron contamination released from high-density dental materials subjected to 6 MV photon beam for a 10 × 10 cm2 field with 90 cm source to surface distance. The materials evaluated were gold alloy, titanium, composites and porcelain, which were defined at 37 mm depth of a phantom. The results indicated an increase in dose, which originated from the electron contamination released from gold alloy and titanium. Due to its importance, it was recommended that this effect should be taken into account in clinical radiotherapy. Farahani et al. [3] investigated dose enhancement in soft tissue in the vicinity of high-density materials, and obtained the lateral dose profiles and percent depth doses by Monte Carlo simulations. The high-density materials evaluated included gold alloy, amalgam alloy, nickel–chromium alloy and natural human tooth. A series of normal and restored teeth in a phantom was irradiated with 10 MV X-ray beam, and a 2.0- and 1.2-fold dose enhancement in tissues before the gold alloy restoration and teeth was observed, respectively. In depths beyond the gold alloy and teeth, due to radiation attenuation in the teeth, the dose enhancement was not significant. In the study conducted by Chin et al. [4], EGS4nrc code was used for the simulation of a 10 × 10 cm2 field of a Varian linac at 97 cm source to surface distance. The tooth was simulated as an 8 × 8 × 12 mm cube at 1 cm depth from the surface of a phantom. Four dental materials, namely, amalgam, Eclipse, enamel and Ceramco in various geometric arrangements, including various air gaps and without air gap, were evaluated. The presence of an air gap reduced the dose enhancement to one-third of its initial value. Unlike the gold alloy, amalgam did not increase the clinically significant backscattering. The insertion of a 3 mm low atomic number material could spare the oral mucus from excess radiation dose. Furthermore, Monte Carlo simulation can provide estimation of the dose from backscattered radiation, and could be used for patient-specific dose calculations in future. Abdul Aziz et al. [5] used BEAMnrc code to simulate 9 MeV electrons of Siemens Primus linac and calculated the distortion in electron dose around the tooth and amalgam for a 10 × 10 cm2 applicator. No significant change in dose at depths was noted before these inhomogeneities and the backscattered dose was less than 2 %. At depths beyond these inhomogeneities, 20.81 and 33.81 % reductions in dose were observed for tooth and amalgam, respectively.
It must be noted that most of these previous studies had examined the dose distribution of photon beams in the presence of tooth or dental restoration materials, and to the best of our knowledge, evaluation of photon contamination released from such materials has not been performed. Thus, the aim of the present study was to evaluate the effect of tooth and various dental restoration materials on the electron dose distribution and production of photon contamination in radiotherapy with electron beams of various energies.
Materials and methods
Monte Carlo simulation validation of linac
Siemens Primus linac was simulated, and the simulations were verified in a previous study [7]. In the present study, the verified simulations were used for dosimetric evaluation of the effect of dental materials. In the previous study, 8, 12 and 14 MeV electron energies of electron mode of Siemens Primus linac was simulated using MCNPX code with 10 × 10, 15 × 15 and 25 × 25 cm2 applicators. The percentage depth dose was calculated based on the simulations of these energies and applicators, and the simulations were compared with the measurements. The comparisons were performed using gamma function, and a good agreement was observed between the simulations and measurements when 3.0 % and 2.0 mm criteria were used in gamma function calculations [7].
Effect of tooth and dental restorations
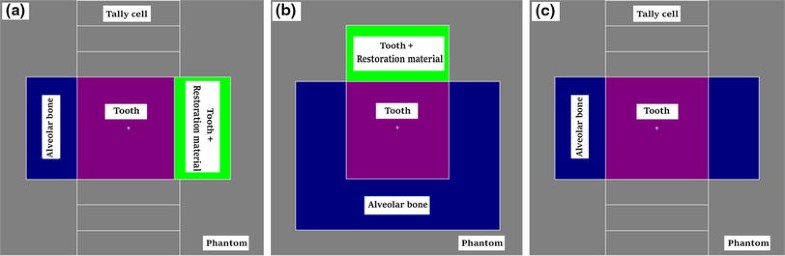
Schematic views of phantom including tooth, and tooth and dental restoration material. Parts (a), (b) and (c) show various cross sections of the phantom, tally cells, alveolar bone, tooth and the restoration material. The central axis of the electron beam is perpendicular to the cross section in part (b)
Composition [percentage weight (%)] of enamel, dentine, Ceramco, Eclipse, amalgam and alveolar bone used in the present study
| Enamel [10] | Dentine [10] | Ceramco [4] | Eclipse [4] | Amalgam [4] | Alveolar bone [10] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Density (g/cm3) | 2.97 | 2.18 | 2.6 | 13.8 | 8.0 | 1.8 | |||||
| Ca | 36.4 | Ca | 30.5 | Na | 8.32 | Au | 52.0 | Ag | 69.3 | Ca | 14.7 |
| O | 41.92 | O | 36.14 | K | 7.07 | Pd | 37.5 | Sn | 17.9 | O | 41.0 |
| C | 1.47 | C | 11.3 | Al | 14.65 | Zn | 4.0 | Cu | 11.8 | C | 27.8 |
| P | 17.5 | P | 15.0 | Si | 15.24 | In | 3.0 | Zn | 1.0 | P | 7.0 |
| H | 0.98 | H | 3.08 | O | 38.97 | Sn | 3.0 | H | 6.4 | ||
| N | 0.13 | N | 2.5 | Sn | 15.76 | Re | 0.5 | N | 2.7 | ||
| Mg | 0.4 | Mg | 1.1 | ||||||||
| Na | 0.6 | Na | 0.2 | ||||||||
| F | 0.01 | F | 0.02 | ||||||||
| Zn | 0.016 | Zn | 0.018 | ||||||||
| Cl | 0.25 | Cl | 0.03 | ||||||||
| K | 0.3 | K | 0.07 | ||||||||
| Fe | 0.003 | ||||||||||
| Cu | 0.01 | ||||||||||
| Si | 0.003 | ||||||||||
A total of 5.0 × 108 electron histories were transported and the maximum uncertainty in Monte Carlo calculations for the tally cells was 5.16 %. The relative electron dose was calculated as the ratio of electron dose in a voxel in the phantom with tooth (or tooth including restoration material) to the electron dose in the same voxel in the phantom without tooth (or tooth including restoration material). The relative electron dose values were compared for various restoration materials, depths and electron energies. Absolute electron doses were obtained while 100.00 cGy dose was prescribed to the depth of maximum dose. It should be noted that the depth of maximum dose depends on the electron energy. In addition, the relative electron dose was also calculated for tooth when the input files were run with 2.0 × 109 electrons, and the results were compared with those obtained when the files were run with 5.0 × 108 electrons. The maximum uncertainty in Monte Carlo calculations was 2.59 % for the case of 2.0 × 109 electron histories. The results of the electron dose calculation using F4 tally (in combination with mass collision stopping power) were compared with dose calculations using F6 and *F8 tallies. The maximum Monte Carlo uncertainty in the calculation of these two later tallies was 5.10 and 5.09 %, respectively. The combined uncertainty is calculated by combination of type A and type B uncertainties. In this study the type B was ignored, and the type A uncertainties were extracted from the Monte Carlo output files. Therefore, the combined uncertainty was equal to type A uncertainty. When a relative dose was calculated, a combined type A uncertainty of the division operation was calculated. Expanded uncertainty was then calculated by multiplication of a cover factor (k) and the combined uncertainty. A cover factor of 2.0 was considered, which equals to a 95.0 % confidence interval.
Photon contamination dose calculation
For the calculation of photon contamination dose in the phantom in the presence of tooth and restoration materials, *F4 tally was used to estimate the photon energy fluence and then multiplied by mass energy absorption coefficients in soft tissue [12]. With 5.0 × 108 and 2.0 × 109 electron histories, the maximum uncertainty in the Monte Carlo calculation was 0.80 and 0.41 %, respectively. The relative photon dose was calculated as the ratio of the photon dose in a voxel in the phantom with tooth (or tooth including restoration material) to the photon dose in the same voxel in the phantom without tooth (or tooth including restoration material). All other simulation details were the same as those described previously for the calculation of relative electron dose. The results of the photon dose calculation using *F4 tally (in combination with mass energy absorption coefficient) were compared with the dose calculations using F6 tally. The maximum Monte Carlo uncertainty in the calculation using these tallies was 0.80 and 0.81 %, respectively. The expanded uncertainties were calculated as described in the previous section, as for calculation of the expanded uncertainties of the electron dose values.
Absolute photon contamination doses (μGy) were also calculated in the soft tissue phantom and for the cases of presence of tooth or dental restoration materials in the phantom for 8, 12 and 14 MeV electron beams. To obtain the absolute photon data, an electron dose of 100.00 cGy was prescribed to the depth of maximum dose in the soft tissue phantom. To obtain the absolute photon dose, it was necessary to calculate photon flux when the electron dose is 100.00 cGy (equal to 100 monitor units) at the depth of maximum dose. To obtain the photon dose, an sphere with 1.0 cm diameter was defined on the central axis of the beam at 99.5 cm distance from the source. Each electron energy was evaluated independently and the input files for calculation of photon flux were run for 3.0 × 107 particles. The maximum type A uncertainty was 1.87 % in these programs. Finally, the absolute photon dose were obtained from the outputs of electron dose (MeV/g), photon dose (MeV/g), photon flux, and electron fux for the case of 100.00 electron dose prescription.
Results
Relative electron dose
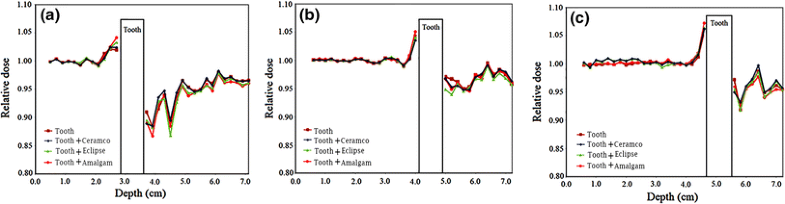
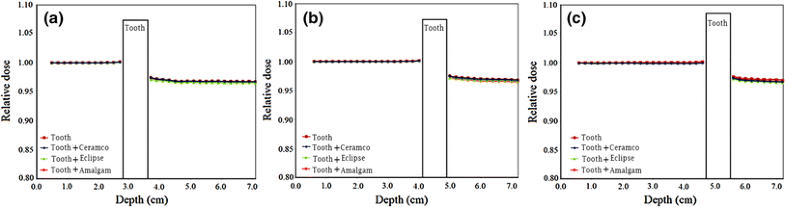
Relative electron dose in the presence of tooth or dental restoration materials in the phantom for 8, 12 and 14 MeV electron beams
Relative electron dose in the presence of tooth or dental restoration materials in the phantom. The values are related to data on the central axis of the beam. The data for 8, 12 and 14 MeV electron energies are presented in parts (a), (b) and (c), respectively
Relative electron dose in the presence of tooth or dental restoration materials in the phantom for 8, 12 and 14 MeV electron beams
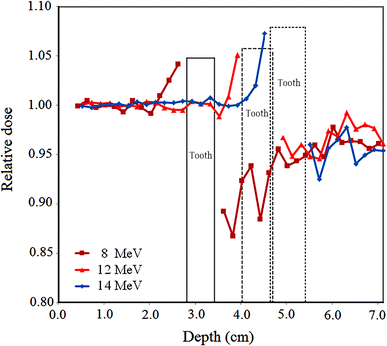
Relative electron dose in the presence of tooth restored with amalgam in the phantom for 8, 12 and 14 MeV electron energies
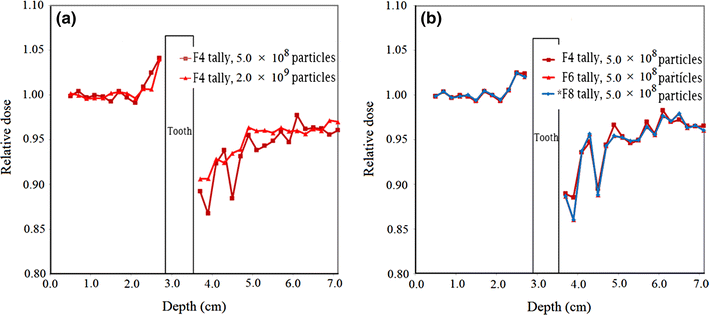
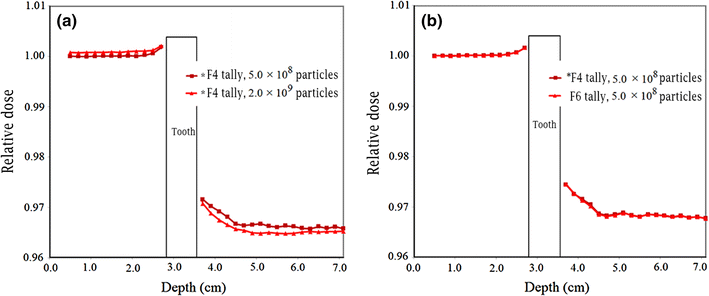
Relative electron dose in the presence of tooth with restoration material in the phantom for 8 MeV electron beam. a Amalgam with run of 5.0 × 108 and 2.0 × 109 electron histories; b Ceramco C3 veneer for run of 5.0 × 108 electron histories with F4 (in multiplication with mass collision stopping power), F6 and *F8 tallies
Relative photon contamination dose
Relative photon dose in the presence of tooth or dental restoration materials in the phantom. The values are related to data on the central axis of the beam. The data for 8, 12 and 14 MeV electron energies are presented in parts (a), (b) and (c), respectively
Relative electron dose in the presence of tooth or dental restoration materials in the phantom for 8, 12 and 14 MeV electron beams
Relative photon dose in the presence of tooth with restoration material in the phantom for 8 MeV electron beam. a Amalgam with run of 5.0 × 108 and 2.0 × 109 electron histories; b Ceramco C3 veneer for run of 5.0 × 108 electron histories with *F4 (in multiplication with mass energy absorption coefficient) and F6 tallies
Discussion
In the present study, the effect of tooth and various dental restoration materials on electron dose distribution and production of photon contamination in radiotherapy with electron beams of a medical linac was evaluated. From Fig. 2, a relative dose higher and lower than 1.00 before and beyond the tooth, respectively, could be observed. As the tooth or tooth restored with a material can be accounted as inhomogeneities with atomic numbers and mass densities higher than those of soft tissue, these effects can be related to backscattering from the tooth and attenuation in the tooth, respectively. The maximum relative electron dose was 1.07 in the presence of tooth including amalgam for 14 MeV electron beam. This equals to 7 % increase in electron dose and is resulted from the electron backscattering from tooth and amalgam. The electron backscattering from the tooth (or tooth and restoration material combination) can increase the dose to the soft tissue before the tooth. Such situation is encountered in radiotherapy of head and neck cancer in the treatment of, for example, cancer of tongue, mandible, maxilla, oral cavity and various types of squamous cell carcinoma. Insertion of a bolus (or another tissue equivalent material which is compatible with the mouth) between the soft tissue and tooth can eliminate this dose enhancement. However, the thickness of such bolus should be selected based on the range of the backscattered electrons in the bolus in each electron energy.
A relative electron dose closer to 1.00 indicates less perturbation in the electron dose in the presence of tooth or tooth with restoration material. Comparison of the relative electron dose values presented in Fig. 2 for various materials revealed that lower electron perturbations are related to tooth, tooth and Ceramco, tooth and Eclipse and tooth and amalgam. This effect can be justified considering the compositions of the materials presented in Table 1. Restoration materials, including an element with high atomic number, which also has a relatively higher weight fraction [e.g. amalgam including 69.3 % of Ag (Z = 47)], have higher electron dose perturbations. While gold in Eclipse and Ceramco has a higher atomic number, when compared with silver in amalgam, it has lower fraction in those two materials (Eclipse and Ceramco) and therefore corresponds to lower electron perturbation.
Considering the change in the trend of relative electron dose with depth in the phantom, it can be observed from Fig. 2 that some fluctuations in the data exist at various depths. These fluctuations can be minimised by running the input files with higher number of electron histories. Another effect is that the relative electron dose is higher and less than 1.00 at points close to the tooth before and beyond the tooth, respectively. This could be related to the range of electrons backscattered or absorbed by the tooth, considering that the electrons have limited ranges in the phantom and therefore affect the dose at the vicinity of the tooth.
The absolute electron doses presented in Table 3 are in agreement with the aforementioned points mentioned on the relative electron dose. Additionally, different phenomena have impact on the data of absolute electron dose, including: electron backscattering from the tooth, electron attenuation from the tooth, and the dose fall off with depth in the phantom. As it can be seen from the data in this table, the electron dose depends on the depth, type of inhomogeneity, and electron energy. Such dependence should be considered in treatment planning of electron beam radiotherapy.
As can be observed from Fig. 3, for a single restoration material and various electron energies, the relative dose for higher energies is higher at depths before and beyond the tooth. This may be the result of higher backscattering and lower attenuation in the tooth at higher energies. However, some exceptions may be observed for this trend.
From Fig. 4a, it can be observed that with the increasing number of particle histories for the same input files, the fluctuations in the data points become less and the relative electron dose data reach closer to the value of 1.00. It is obvious that a higher number of particle histories correspond to a lower Monte Carlo statistical uncertainty; therefore, running the input files with a higher number of particle histories could be advantageous. Due to the relatively large number of situations being evaluated in this study, a number of computers with high processing capabilities may be needed. Figure 4b also implies that that the results of relative electron dose obtained using F6 and *F8 tallies are comparatively the same. While the results obtained using F4 tally are, to some extent, different at depths beyond the tooth, they are very close to the data obtained using other tallies in other points. The Monte Carlo statistical uncertainty with various tallies was practically the same when the input programs were run with the same particle histories (maximum uncertainties of 5.16, 5.10 and 5.09 % with F4, F6 and *F8 tallies, respectively). With the aim of having lower uncertainty in the calculation of the quantity of electron dose, all the tallies exhibited the same preferences.
From the data presented in Fig. 5, it can be observed that the relative photon dose associated with photon contamination is rather equal to and lower than 1.00 before and beyond the tooth, respectively. Furthermore, no considerable difference was noted between the relative photon dose for various materials and electron energies. As shown in Fig. 6a, a comparison between the data obtained with 5.0 × 108 and 2.0 × 109 electrons indicated that the values obtained with higher particle scoring were higher than those obtained with 5.0 × 108 particles at depths before the tooth. Conversely, a reverse effect was noted beyond the tooth. As indicated in Fig. 6b, *F4 (in combination with mass energy absorption coefficient) and F6 tallies showed rather the same relative photon contamination doses. With regard to the calculation of the relative photon dose, while various tallies exhibited practically the same uncertainties, they also presented similar relative doses, and therefore, there may not be any preference for a particular tally in this case.
The data presented in Table 4 indicates that with prescription of 100.00 cGy electron dose to the depth of maximum dose, the absolute photon contamination values are in the range of ~108–376 µGy. These values are not clinically significant. These data have minor variations with depth and type of inhomogeneity. On the other hand the photon contamination is more at higher electron energies. There are also other studies on evaluation of photon contamination in clinical electron beams [13, 14].
A comparison of the results of the present study, with the other studies may be interesting. However, to the best of our knowledge there is not any study with the same scope as it is herein, including the same materials and electron energies. Shiu and Hogstrom [6] evaluated the effect of bone and electron dose distribution 7, 10, 15 and 18 MeV electron energies, based on in-phantom measurements using parallel plate chamber, radiochromic film and thermoluminescent dosimeter. Since there are differences between the methodologies of these two studies, it is not rational to perform an quantitative intercomparison between these two studies. However, the obtaines results in both studies show that there is electron backscattering and attenuation from the inhomogeneity (tooth in this study and bone in the study by Shiu and Hogstrom).
This study was only evaluated the dose distribution along the central ray-line through tooth or the dental restoration materials. It is mainly examining the backscattered dose enhancement and the attenuation of the tooth. It would be interesting to evaluate the level of dose variation in two-dimensional or three-dimensional due to presence of tooth and tooth with restoration materials. It was tried to obtain such information herein but the related uncertainties (in tally voxels of normal dimensions of 2 mm × 2 mm × 2 mm) were high and variance reduction methods should be used to have an acceptable uncertainty level. This could be a subject of a further study on this field.
Conclusion
Based on the results obtained in the present study, the presence of tooth and dental restoration material changes the electron dose distribution and photon contamination in phantom, depending on the type of the restoration materials and electron beam’s energy. Therefore, it can be recommended that the change in the electron dose distribution should be taken into account in the treatment planning systems when teeth are irradiated in electron beam radiotherapy of head and neck cancer. Furthermore, to some extent, the various effects observed for different restoration materials and electron energies could be explained. If treatment planning is performed in such a way that the teeth are excluded from primary irradiation, potential errors in treatment planning and higher dose irradiation of the normal tissues adjacent to the teeth could be avoided. However, based on the restoration materials and geometries evaluated, photon contamination in presence of restoration materials are not clinically significant.
Notes
Acknowledgments
The authors would like to thank Mashhad University of Medical Sciences (MUMS) for funding this work.
References
- 1.Reitemeier B, Reitemeier G, Schmidt A, Schaal W, Blochberger P, Lehmann D, Herrmann T (2002) Evaluation of a device for attenuation of electron release from dental restorations in a therapeutic radiation field. J Prosthet Dent 87(3):323–327CrossRefPubMedGoogle Scholar
- 2.Bjelkengren U (2007) Absorbed dose distributions in the vicinity of high-density materials in head and neck radiotherapy: a quantitative comparison between measurements, Monte Carlo simulations and treatment planning system. MSc Thesis in Medical Radiation Physics Clinical Science, Lund University. http://www.lunduniversity.lu.se/o.o.i.s?id=24965&postid=2157049
- 3.Farahani M, Eichmiller FC, McLaughlin WL (1990) Measurement of absorbed doses near metal and dental material interfaces irradiated by X- and gamma-ray therapy beams. Phys Med Biol 35(3):369–385CrossRefPubMedGoogle Scholar
- 4.Chin DW, Treister N, Friedland B, Cormack RA, Tishler RB, Makrigiorgos GM et al (2009) Effect of dental restorations and prostheses on radiotherapy dose distribution: a Monte Carlo study. J Appl Clin Med Phys 10(1):80–89CrossRefGoogle Scholar
- 5.Abdul Aziz MZ, Yusoff AL, Salikin MS (2011) Monte Carlo electron beam dose distribution near high density inhomogeneities interfaces. World Acad Sci Eng Technol 58:338–341Google Scholar
- 6.Shiu AS, Hogstrom KR (1991) Dose in bone and tissue near bone-tissue interface from electron beam. Int J Radiat Oncol Biol Phys 21(3):695–702CrossRefPubMedGoogle Scholar
- 7.Bahreyni Toossi MT, Ghorbani M, Akbari F, Sobhkhiz Sabet L, Mehrpouyan M (2013) Monte Carlo modeling of electron modes of a Siemens Primus linac (8, 12 and 14 MeV). J Radiother Pract 12(4):352–359CrossRefGoogle Scholar
- 8.Waters LS. MCNPX user’s manual, version 2.4.0. Report LA-CP-02-408 2002; Los Alamos National LaboratoryGoogle Scholar
- 9.The International Commission on Radiation Units and Measurements. Tissue substitutes in radiation dosimetry and measurement. ICRU Report 44. Bethesda, MD (1989)Google Scholar
- 10.Shved VA, Shishkina EA (2000) Assessment of tooth tissues dose rate coefficients from incorporated strontium-90 in EPR dose reconstruction for the Techa Riverside population. In: Harmonization of radiation, human life and the ecosystem, Proceedings of 10th international congress on radiation protection. International Radiation Protection Association, Hiroshima; CD-ROM; Paper No. P-3a-212Google Scholar
- 11.National Institute of Standards and Technology (NIST) (2014) http://physics.nist.gov/cgi-bin/Star/edata.pl
- 12.National Institute of Standards and Technology (NIST) (2014) http://physics.nist.gov/PhysRefData/XrayMassCoef/ComTab/tissue.html
- 13.el-Khatib EE, Scrimger J, Murray B (1991) Reduction of the Bremsstrahlung component of clinical electron beams: implications for electron arc therapy and total skin electron irradiation. Phys Med Biol 36(1):111–118CrossRefPubMedGoogle Scholar
- 14.Sharma AK, Supe SS, Anantha N, Subbarangaiah K (1995) Physical characteristics of photon and electron beams from a dual energy linear accelerator. Med Dosim 20(1):55–66CrossRefPubMedGoogle Scholar